It is undoubtedly the most used over-the-counter medicine by parents of very young children. But be careful, there are some boxes of Doliprane 2.4% oral suspension for children and infants a manufacturing defect that could lead to overdose.
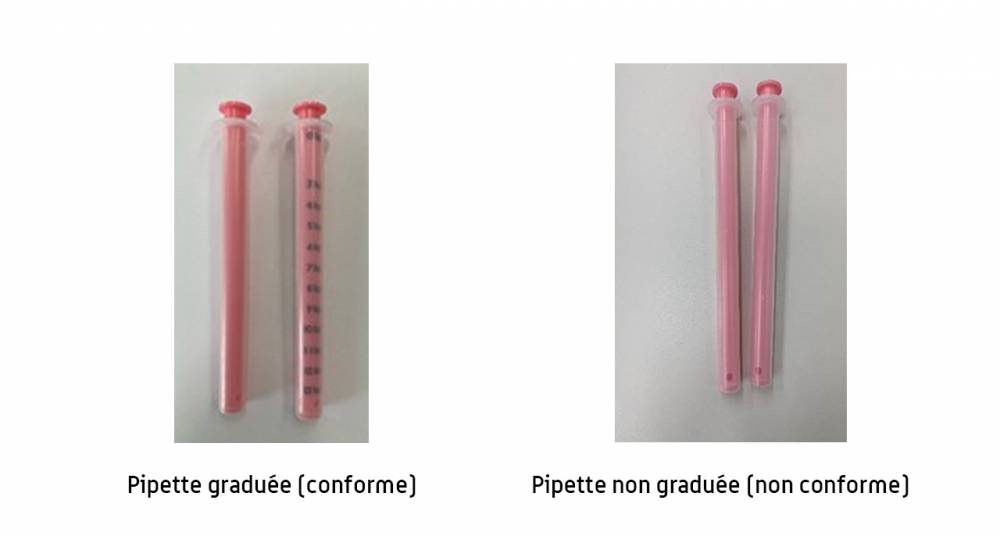
This was indicated yesterday, Thursday 16 May, by the National Agency for the Safety of Medicines (ANSM) in a press release. The ANSM was informed by the Opella Healthcare France laboratory which markets pediatric Dolipraneabout thirty pipettes used to withdraw the right dose were without graduations.
Defective products are contained in the following lots: 3KLR12D1, 3KLR13D2, 3KLQ70DU, 3KLQ69DT, 3KLR14D3, 3KLQ71DV, 3KLQ72DW. That is 1.3 million boxes affected.
“If you have a box of Doliprane 2.4% oral suspension, check that the pipette is graduated correctly », warns the ANSM. If there is a graduation, which indicates the child’s weight in the form of lines, it can be used.
“The defect only concerns the pipette. The integrity and quality of the syrup contained in the bottle are intact”the health authority further specifies.
What to do if the Doliprane pipette is defective?
If you have a bottle of Doliprane whose pipette is not graduated, you can return it to the pharmacy, where it will be exchanged. “Do not use the non-graduated pipette or the pipette of another drug, as this may cause a dosing error”warns the ANSM.
Furthermore, if you purchase a box of Doliprane 2.4% oral suspension from one of the affected lots, the pharmacist will check the state of the pipette in front of you at the time of delivery.
Listen to Laisse-moi kiffer, Madmoizelle’s cultural advice podcast.
Source: Madmoizelle
Mary Crossley is an author at “The Fashion Vibes”. She is a seasoned journalist who is dedicated to delivering the latest news to her readers. With a keen sense of what’s important, Mary covers a wide range of topics, from politics to lifestyle and everything in between.




