Invited on the set of “4 Vérités” (France 2) on Thursday 7 March, the Minister of Health, Catherine Vautrin, announced that the government wants to allow Social Security to fully cover a saliva test to detect endometriosis, starting from January 2025.
The cost of this endotest », designed by the French biotechnology company Ziwig, is now estimated at 1000 euros. “ We still have a trial in 3,000 women until the end of the year and the evidence is extremely interesting » argued the minister on the set of France 2. How does the test work? What are the criteria to be admitted? How is it administered?
Answers with Ziwig founder Yahya El Mir.
To miss. How does the test work?
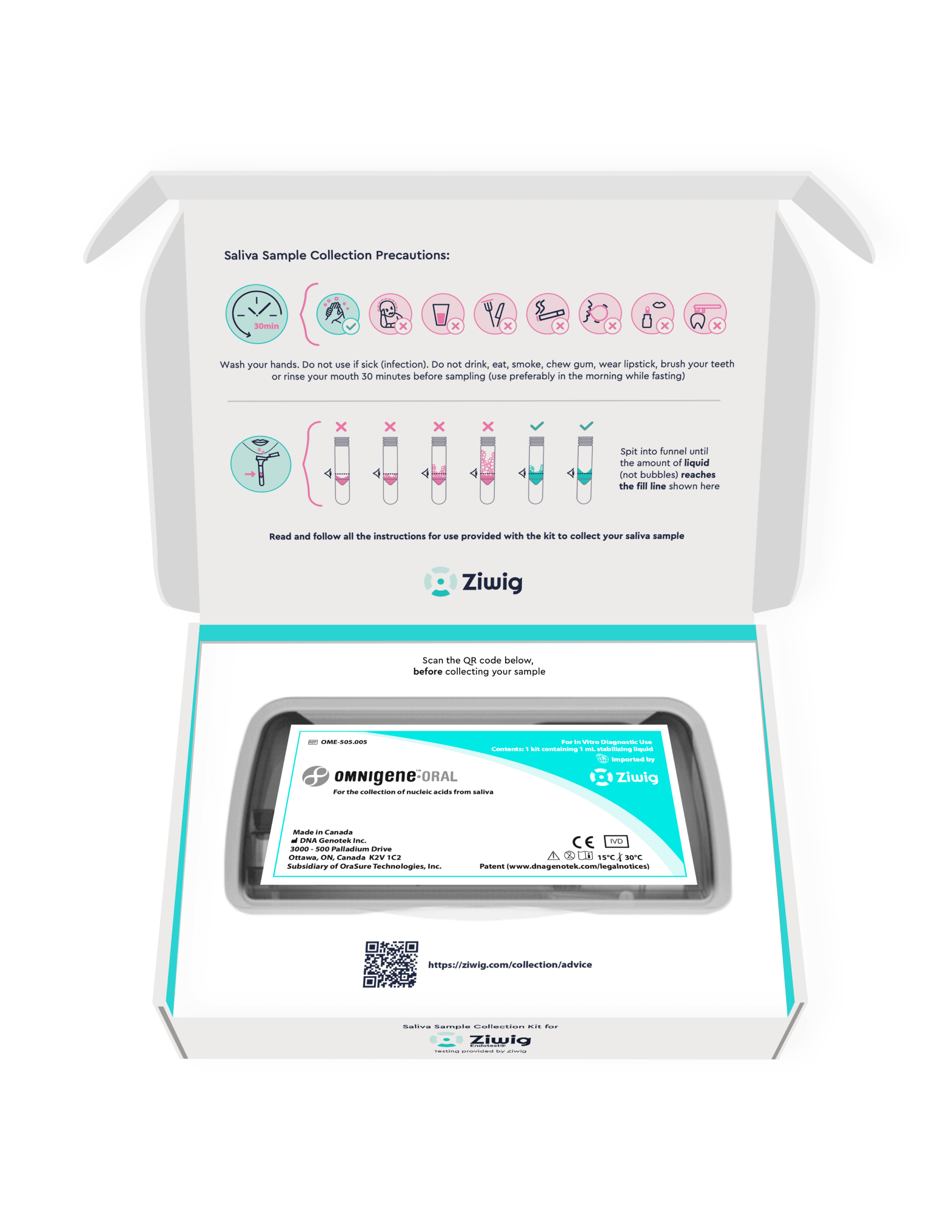
Yahya El Mir. This is a test done with two microdrops of salivain which we will examine RNA and identify biomarkers of endometriosis.
The technology we developSalivary RNA analysis, has immense potential for health. When I started to discover this, I gathered my teams and told them: we will go where the need is most: women’s health. This is where we will have a real impact on patients’ lives. We have received awards and have been published in the most prestigious scientific journals. And what touched us most, as a team, was reading the first testimonies of women who finally, after sometimes thirty years of pain, infertility and misunderstanding, had a name to put on record as the cause of their suffering. This is what makes us most proud.
How is the test carried out?
This is not a self-test. The test must be prescribed by a doctorand the sample is processed in a specialized laboratory. But it is simple, safe and non-invasive.
The test will be distributed through a network of partner laboratories.
Who can benefit from it?
Affected patients must be between the ages of 18 and 43 and have symptoms suggestive of endometriosis. Once the test is done, the diagnosis can be made in about ten days.
When we know that it takes women an average of ten years to get diagnosed, this is a big step for their health!
Is it already on the market in other countries?
Yes it is on the market in 17 countries. We got the Certification CE, this is a marketing authorisation. This test is the result of several cutting-edge technological innovations, and if we put it on sale today in France, all the women who need it would not be able to access it, due to its cost, and it would be very unfair. For this reason we turned to the High Authority of Health (HAS), to start a reimbursement procedure, to guarantee equal access to this test for women.
In this context, HA confirmed clearly and sustainably the effectiveness and performance of the testand required an additional study to evaluate in which phase of patient care it was most relevant to use it. We are already well advanced in this process and Minister Catherine Vautrin has set a goal: reimbursement for all women in need in 2025.
How reliable is it?
The diagnostic performance of Ziwig Endotest® communicated by the High Health Authority (HAS) in its opinion of 8 January 2024, is high, with a sensitivity of 95% and a specificity of 94%. The performance of Ziwig Endotest was confirmed by a multicenter study (18 centers) on more than 1,000 people, the results of which were shared with the HAS, as part of the reimbursement process.
More articles on
endometriosis
-
Cancer has left me with an invisible handicap and I’m tired of having to constantly justify myself
-
To “demographically rearm” France, government is considering a fertility assessment at age 25
-
At 37 they removed my uterus and it was the best decision
-
A saliva test to detect endometriosis soon available?
-
The National Rally withdraws the bill on endometriosis
Discover BookClub, Madmoizelle’s show that questions society through books, in the company of those who make them.
Source: Madmoizelle
Mary Crossley is an author at “The Fashion Vibes”. She is a seasoned journalist who is dedicated to delivering the latest news to her readers. With a keen sense of what’s important, Mary covers a wide range of topics, from politics to lifestyle and everything in between.




